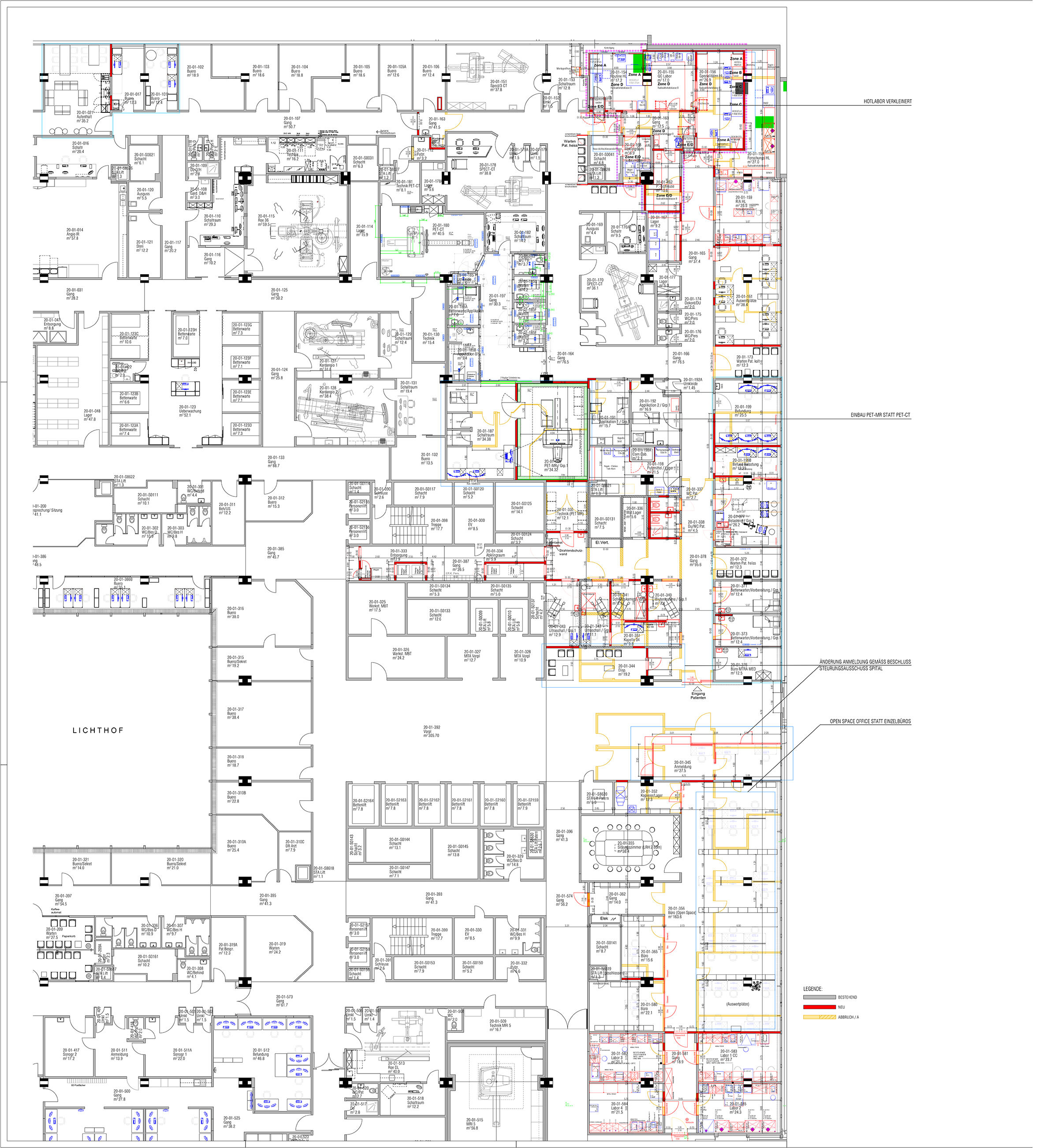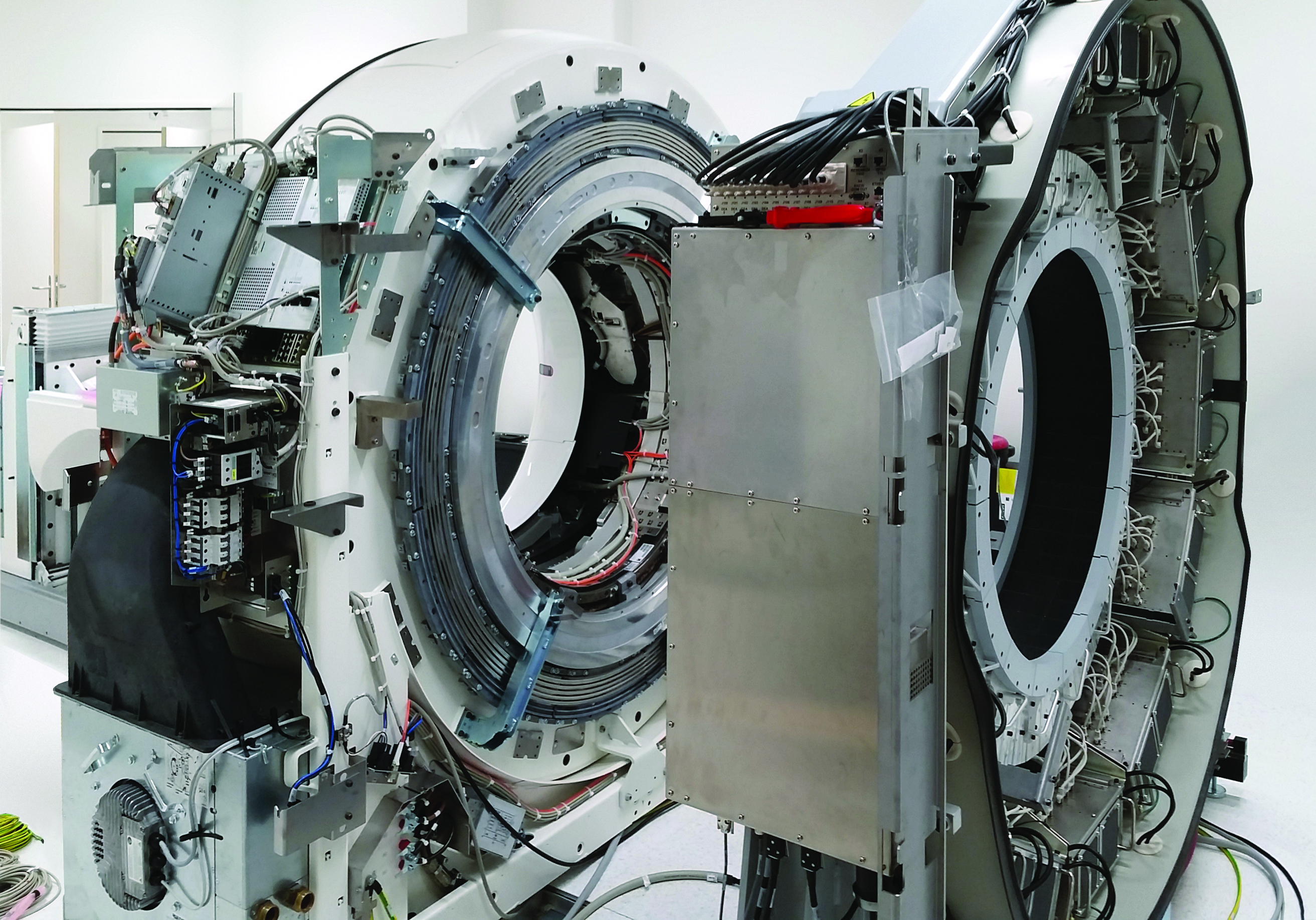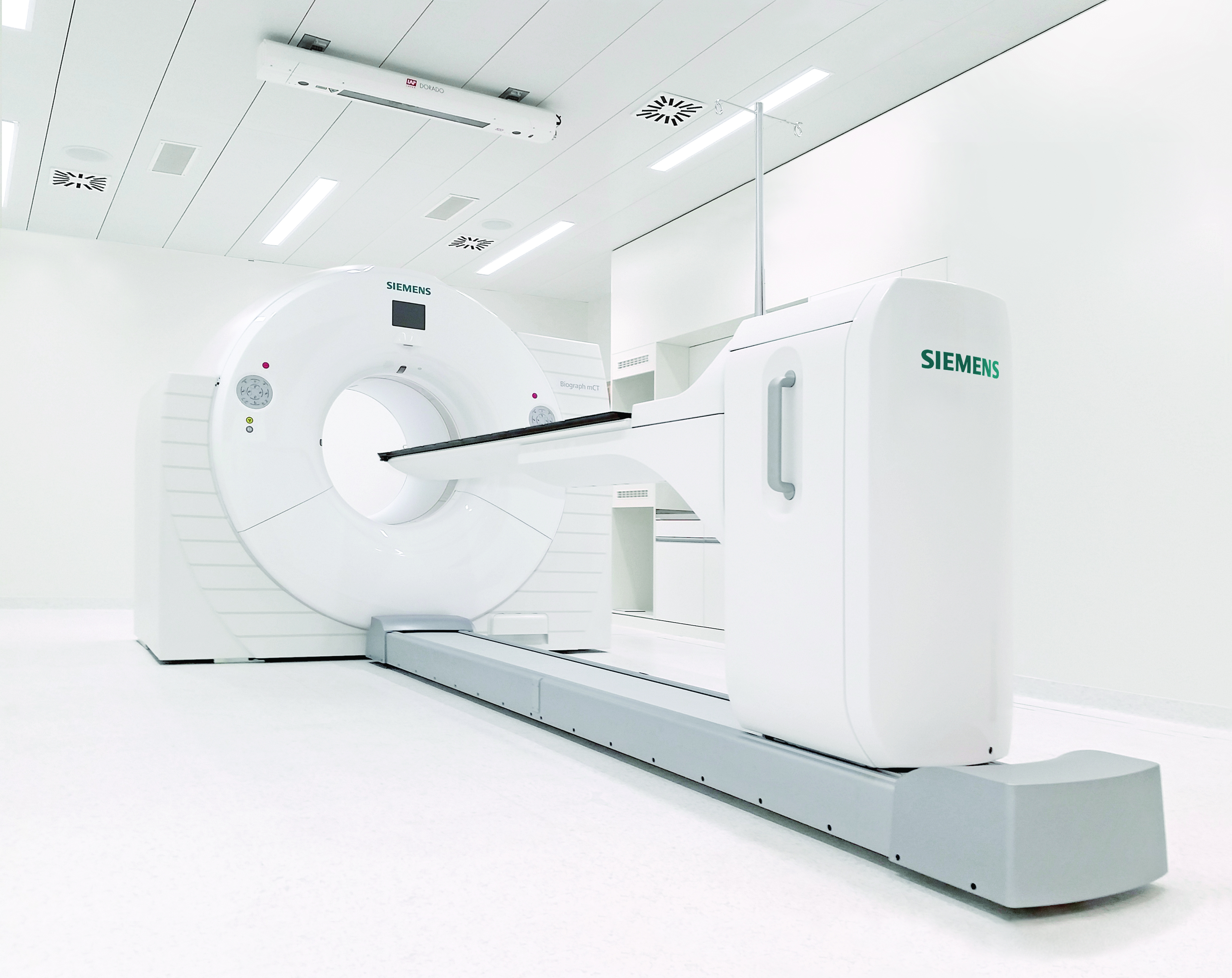
The conversion was carried out in five stages while the facility was still in operation. The trigger for the reconstruction was the construction of a connecting corridor to the new OR East. This separated the previously closed, controlled area of the nuclear medicine and radiopharmaceutical chemistry departments roughly in the middle.
Requirements of "Good Manufacturing Practice" (GMP) and the "current Guidelines on Good Radiopharmacy Practice" (cGRPP; =guidelines of the EANM (European Association of Nuclear Medicine), which were adopted and put into force by the Swiss authorities (BAG and Swissmedic), necessitated the establishment of a clean room for the manufacture of radiopharmaceuticals.